Our Research
The research in our laboratory is focused on application of the tools of engineering, glycobiology and molecular biology to increasing the understanding vascular disease. Our long-term goals are to improve implanted vascular device design, develop technology to enable high-throughput study and drug discovery in the cardiovascular field and to enhance cellular and protein therapies for ischemic disease. We are interested in four main areas:
Vascular Mechanotransduction
We are interested in understanding how vascular cells sense and respond to mechanical forces. In particular, we focus on the role of cell surface glycoproteins in vascular cell mechanotransduction to shear stress and mechanical stress. We utilize novel devices to the control local mechanical environment in cultured cells and combine these devices with powerful molecular tools such as lentiviral gene delivery and in-vivo gene knockout models.
Therapies for Myocardial and Peripheral Ischemia
Chronic myocardial ischemia affects more than 27 million patients in USA and is the leading cause of morbidity and mortality in developed countries. Peripheral ischemia is even more prevalent, occurring in 12-20% of the population aged 65 and older in USA. One appealing and powerful strategy for treating ischemia would be to revascularize the tissue by stimulating the development of native vasculature. While laboratory studies in the past have shown effectiveness of growth factors, growth factor genes and stem cells in resolving ischemia by promoting angiogenesis in the tissue. However these treatments have had only limited effects when used in a clinical setting after getting positive results in animal models. Our studies focus on understanding why diseased tissues are resistant to current therapies and aims to develop novel protein and cell based methods to treat ischemia
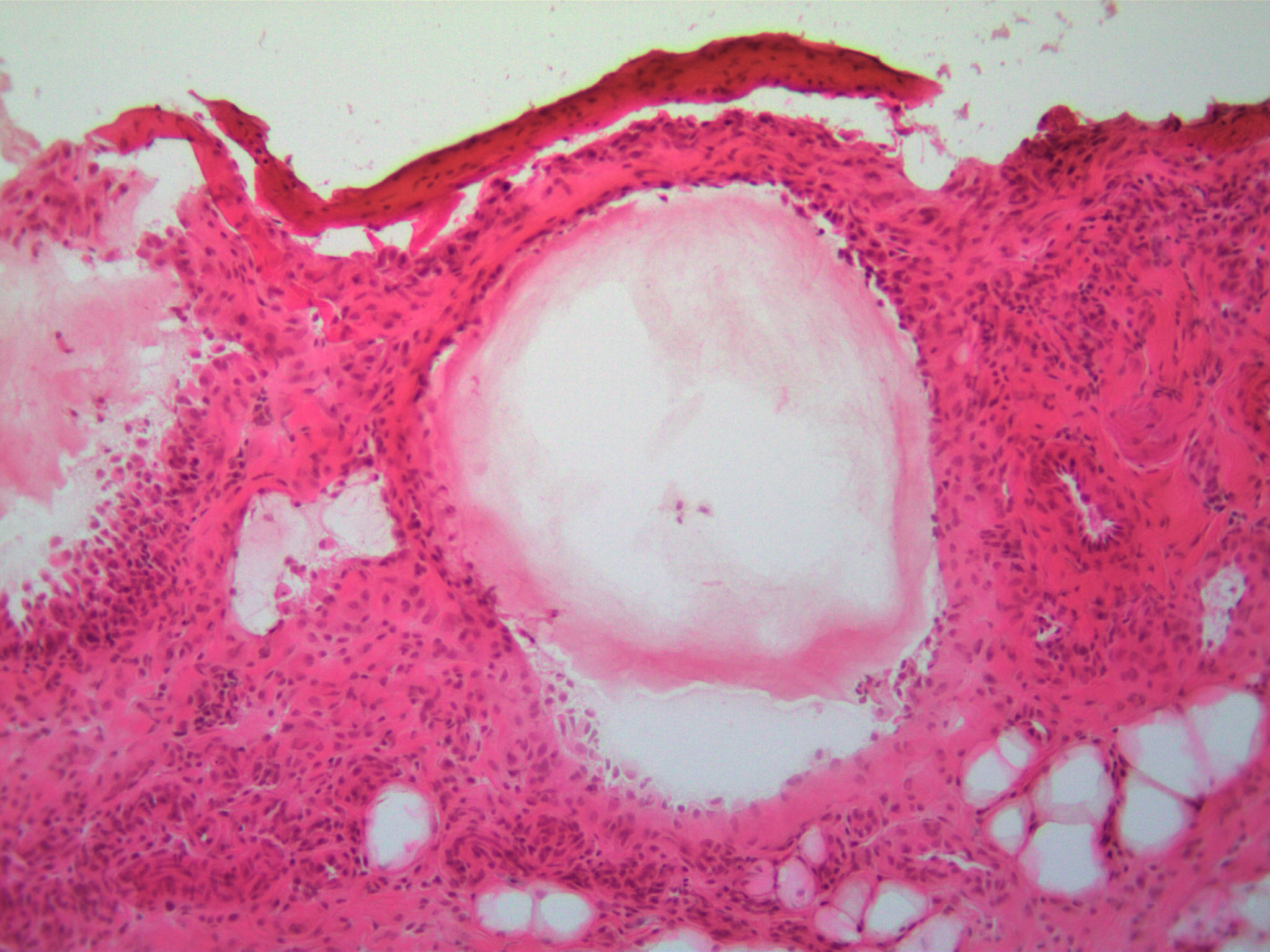
Mechanisms of Atherosclerotic Plaque Formation and Rupture
Atherosclerosis is a pathophysiological process that underlies many types of cardiovascular disease. It is a long-term chronic disease that causes the gradual accumulation of lesions in the arteries containing lipid deposits, inflammatory cells and proliferating vascular cells. As these plaques grow and develop, they can narrow the arteries leading to a reduction in blood flow and, consequently, the development of ischemia in the heart and other organs. In addition, some of the plaques can develop unstable morphology that is prone to sudden rupture and release of lipids into the blood, leading to acute thrombosis and occlusion of the vessel causing heart attack, stroke and embolus. Our work in this area focuses on glycoproteins and glycoprotein metabolism in controlling plaque formation and rupture.
Implanted Medical Devices
While clinical interventions such as endovascular stents are often able to provide immediate improvements in tissue perfuse, these devices themselves are often limited by the local tissue response and vascular remodeling. We use a combination of molecular techniques and novel animal models to examine the mechanisms of restenosis and thrombosis in the context of implanted vascular devices. We are particularly interested in understanding the role of novel glycoproteins in this context using this knowledge to develop strategies for improving current device design.
Our Collaborators
Functional Optical Imaging Laboratory, UT Austin
Ultrasound Imaging and Therapeutics Research Laboratory, UT Austin
Laboratory for Cardiovascular Tissue Engineering, UT Austin
Texas Cardiac Arrhythmia Institute, St. David's Medical Center
Seton Institute of Reconstructive Plastic Surgery
Dell Children's Pediatric Medical Center: Dr. Patrick Kelley
Dr. Elazer Edelman's Laboratory, Massachusetts Institute of Technology
Dr. Peter Stone's Laboratory, Brigham and Women's Hospital
Dr. Matthew Nugent's Laboratory, Boston University
Vascular Biology Research Laboratory, Massachusetts General Hospital